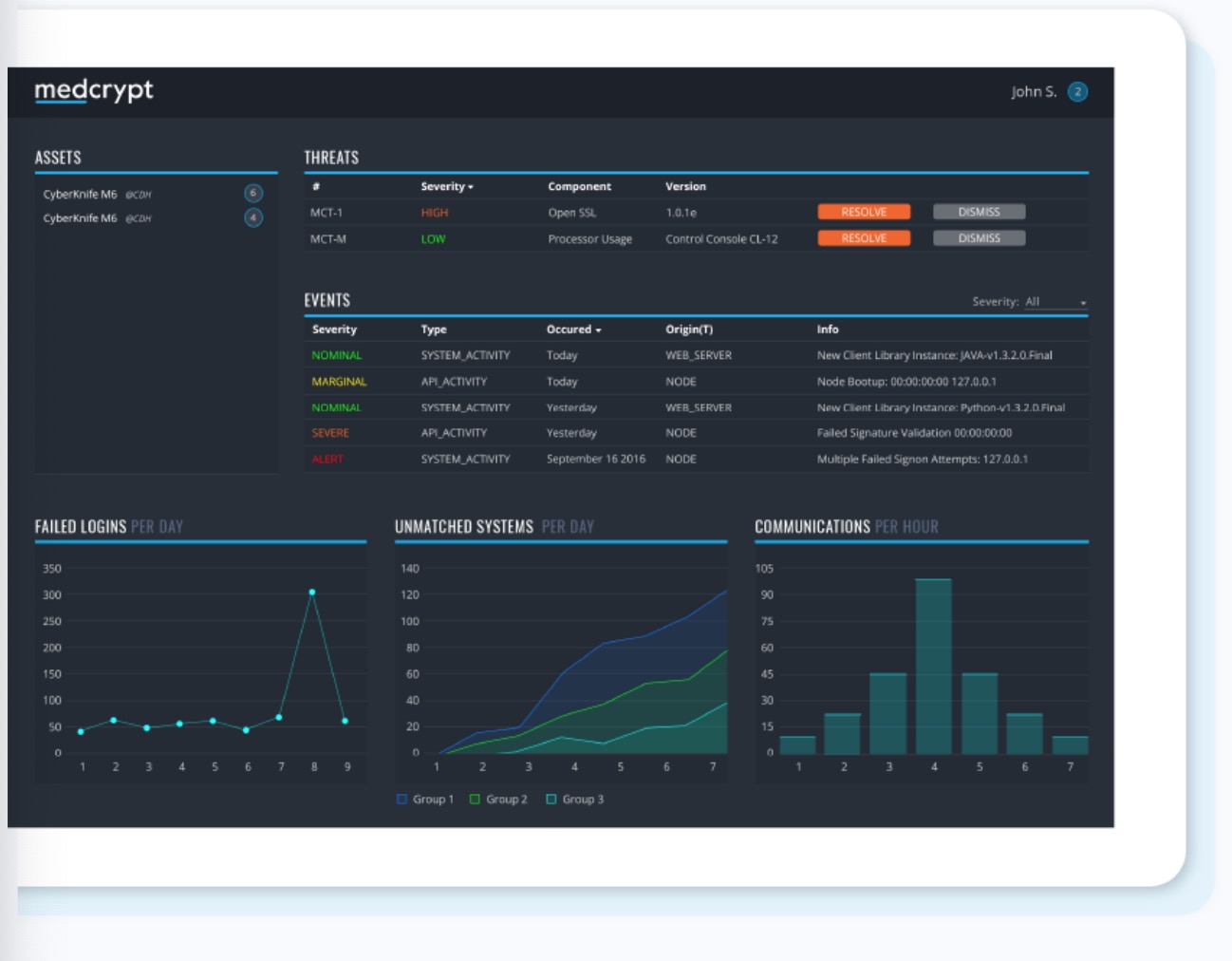
What You Should Know:
– MedCrypt, the proactive cybersecurity solution provider for medical devices and manufacturers, will announce the close of a Series B extension round led by Dexcom Ventures, bringing the company’s total to date to $36.4M.
– With support from three major investors, Johnson & Johnson Innovations, Intuitive Ventures, and Dexcom Ventures, MedCrypt continues to lead the way in developing cybersecurity technologies and infrastructure for life-saving medical devices in all facets of healthcare, including the diabetes industry. Approximately 37.3 million Americans live with diabetes, meaning there are high numbers of insulin and glucose monitors being used.
Cybersecurity Hurdles for Connected Health Devices
The global wearable medical device market size is currently valued at $21.3 billion and is expected to grow 28% by 2030. Cybersecurity remains one of the biggest hurdles for connected devices across all areas of healthcare, including the diabetes industry. The Food and Drug Administration (FDA) tracked the potential cybersecurity risks associated with both insulin and glucose monitors, noting these devices need to be reliable and trustworthy as users are pairing them with other system components such as smartphones.
“Diabetes care management is one of the main categories of healthcare innovation that is highly dependent on connectivity; consequently, it is one of the main categories heavily scrutinized by the FDA,” said Mike Kijewski, CEO of MedCrypt. “The promise of software in a device can only be achieved when the cybersecurity posture of the device is secure and effective in the threat landscape. MedCrypt has built a cybersecurity platform for all medical devices – for things as small as pacemakers and insulin pumps or as large as surgical robots – and we are honored to be backed by Dexcom Ventures as we continue our push for better device security.”
Recent Milestones
In 2022, MedCrypt deployed partnerships with 18 new medical device manufacturers that are committed to improving cybersecurity in healthtech. These additional funds will be used to grow MedCrypt’s engineering team to support this growing demand for cybersecurity in devices – across all products – remaining aligned with requirements set by the FDA and the cybersecurity provisions in the latest Consolidated Appropriations Act, 2023.
